Portfolio
All my servers run on Ubuntu Linux, and I stick with a LAMP stack (using MariaDB instead of MySQL) for my SilverStripe and WordPress sites. I don’t touch the content or design on the WordPress sites, but I keep everything running smoothly behind the scenes—updating software, securing configurations, and making sure everything’s locked down with HTTPS.Backups are handled with DigitalOcean snapshots, so I can roll back if anything goes wrong. I’m also working on rolling out mTLS for extra security on admin areas. For code, I use git to keep everything organized and versioned.
Website Development
I have developed websites using SilverStripe, including my own portfolio and a site for Willow Pond Farm, where I collaborated closely with their content team. More recently, I have begun building web applications with Django, such as an asset management system that leverages QR codes to simplify item tracking. This system allows users to access detailed information about assets directly from their devices, without needing to install any additional apps.While my primary focus is on back-end development, I am also capable of front-end UI/UX customization. To ensure my websites are both accessible and responsive, I select high-quality templates created by front-end designers, prioritizing those with strong accessibility and mobile-friendly features.
Software experience
-
SOLIDWORKS
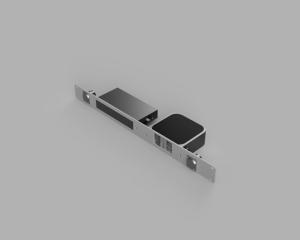
Custom rack mount designed in Fusion360
- Fusion 360
- SAP
- Python
- c++
- Java
Equipment experience
- Automated Filling line
- CVC 300 Wrap-Around Labeler
- CVC 350 Horizontal Ampule/vial Labeler
- Monobloc Filling machine
- Autoclave
- Vacuum chamber
Nova Biomedical
Responsible for performing filling, sealing, and labeling and packaging of various ampule and vial controls by means of written and/or verbal instruction.
Responsible for recording all pertinent information as required in various assembly procedures and logs.
Set up per procedure and equipment specification for controls filling operation. Operating and monitoring of controls filling equipment - load/unload product, assure product is properly filled, sealed, labeled, and quarantined as required.
Documentation - accurate and complete record of control filling and packaging procedure Perform in-process testing and tonometry during filling process.
Periodic maintenance/calibration work orders.
Mechanical aptitude.
Knowledge of Quality System Regulations (QSR).
Knowledge of OSHA safety standards.
Knowledge of Good Manufacturing Practice (GMP).
Skills and Competencies: Ability to work with hand tools and gauges to operate filling/packaging equipment and sterilizers/autoclave.
Willingness to learn ability to adapt to change, and a positive attitude.
Able to work as part of a team or individually.
IDEXX
- Read and interpret manufacturing and quality control documents, technical procedures, and government regulations.
- Responsible for recording all pertinent information as required in various procedures and logs.
- operation of a variety of machines on a rotating basis. This will includes set-up operation, clean-up, changeover, troubleshooting, and/or minor repair for manufacturing equipment
- provided production & process training to other employees.
- working as a team